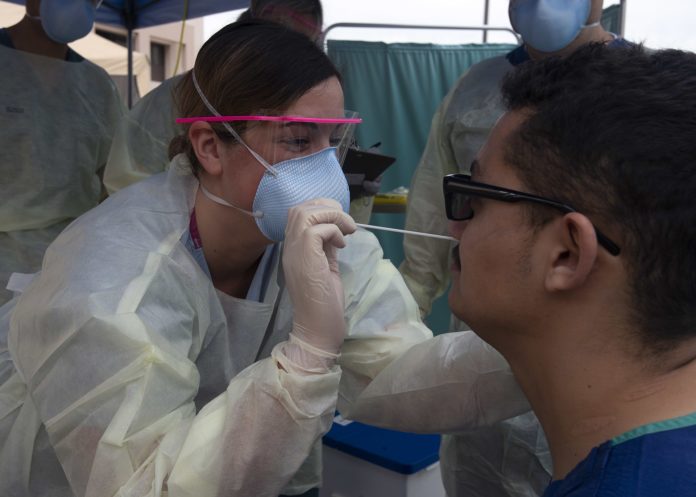
Researchers are working around the clock, innovating ways to improve the COVID-19 testing process. Recently, a group of researchers from Yale University presented findings that suggest that one improvement could be made right at the beginning of the testing process — in the choice of the sample to be tested. Although the current gold standard is to use samples collected from nasopharyngeal swabs (NPS), the group found that using saliva for SARS-CoV-2 detection is more sensitive and consistent than using a nasopharyngeal swab. They assert that saliva should be considered as a reliable sample type to improve COVID-19 testing.
The work is published as a preprint in MedRxiv. The title of the preprint (a paper that has not yet gone through the peer review process) is “Saliva is more sensitive for SARS-CoV-2 detection in COVID-19 patients than nasopharyngeal swabs.”
Currently available SARS-CoV-2 detection tests suffer from multiple challenges including low sensitivity and reliance on swabs. In addition, swab collection requires close contact between the healthcare worker performing the collection and the patient, which poses a health risk to healthcare workers. Using saliva for COVID-19 testing has several advantages when compared to collection of nasopharyngeal swabs. Saliva collection does not require specialized swabs or tubes, is far less uncomfortable for the patient, and may even be done independently by the patient – potentially alleviating the current overwhelming demand for healthcare workers. Indeed, in this study, the saliva samples were self-collected by the patient. The process is described as, “upon waking, patients were asked to avoid food, water, and brushing of teeth until the sample was collected. Patients were asked to repeatedly spit into a sterile urine cup until roughly a third full of liquid (excluding bubbles), before securely closing it.”
The researchers evaluated SARS-CoV-2 detection in paired nasopharyngeal swabs and saliva samples collected from COVID-19 inpatients and asymptomatic healthcare workers at moderate-to-high risk of COVID-19 exposure. They collected clinical samples from 44 COVID-19 inpatient study participants — all of whom had serious illness — and 98 health care workers. After testing for the presence of the SARS-CoV-2 virus, the findings suggest that saliva samples provided greater detection, sensitivity, and consistency throughout the course of an infection than the nasopharyngeal swabs…
To read the entire article from Genetic Engineering & Biotechnology News, click https://www.genengnews.com/news/sars-cov-2-detection-in-saliva-samples-is-sensitive-and-consistent/

