Reports of a respiratory virus spreading in Wuhan, China, emerged in late 2019. After the number of cases outside China increased rapidly, the World Health Organization declared the novel coronavirus a pandemic on March 11.
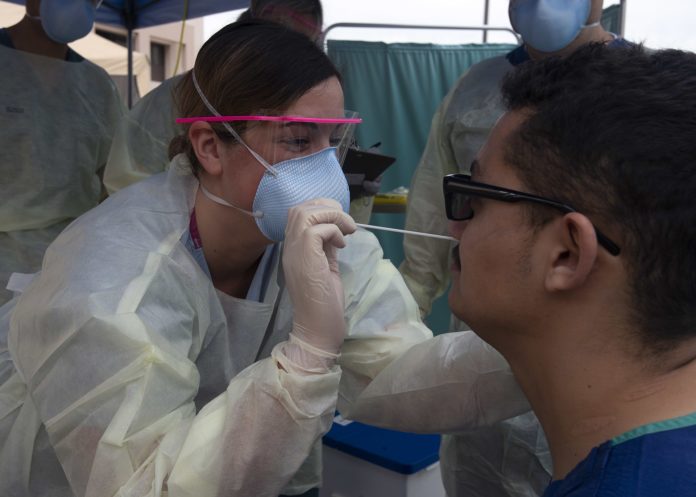
With no cure or vaccine currently available, diagnostic testing is crucial to containing the spread of the virus.
Opportunities to mitigate the pandemic’s impact in the United States were lost amid bureaucratic delays and a breakdown in efforts to produce a reliable test kit at the Centers for Disease Control and Prevention.
Here are the major developments to understand what happened:
1. Contaminated tests
The failure by the CDC to quickly produce a test kit to detect the coronavirus was triggered by a glaring scientific breakdown at the CDC’s lab in Atlanta. The CDC facilities that assembled the kits violated manufacturing practices, resulting in contamination of one of the three test components used in the detection process. The troubled segment of the test was not critical to detecting the novel coronavirus, experts said. After false-positive results emerged, CDC officials took weeks to remove the unnecessary step from the kits.
2. Early warning signs on flawed testing ignored
Trump administration officials continued to rely on flawed CDC tests even as many lab scientists eager to help grew increasingly alarmed and exasperated by the federal government’s actions, according to emails and documents reviewed by The Washington Post. Scientists at academic, hospital and public health labs were frustrated by the bureaucratic demands that delayed their attempts to develop alternatives to the CDC test…
To read the entire article from The Washington Post, click https://www.washingtonpost.com/investigations/2020/04/18/timeline-coronavirus-testing/

